F. Acids and Bases
➢ Solution is acidic if it contains a lot of $H^{+}$
➢ Solution is alkaline if it contains a lot of OH-
➢ Measured on pH scale
- Logarithmic
- Numbered 1-14
■ $\text{Acids 1-7 pH}$
■ $\text{Bases 7-14 pH}$
➢ Buffers maintain stable pH
G. Organic Molecules
➢ Organic compound contains Carbon
➢ Inorganic compound does not contain carbon
➢ Carbon often surrounded by hydrogen
➢ Carbon is a versatile atom
- Can bind with many elements
- Many “slots” to bind with elements
■ 4 valence electrons
● Can form 4 covalent bonds
■ Makes large, complex molecules possible - In molecules with multiple carbons, each carbon bonded to 4 other atoms has a tetrahedral shape
■ When 2 carbons are formed by a double bond, the atoms joined to the carbons are one the same plane as the carbons - Electron configuration gives it covalent compatibility with other elements
- Hydrocarbons consist of only carbon and hydrogen
■ Can undergo reactions that release a large amount of energy
■ isomers are compounds with the same molecular formula but different
structures/properties
● Usually only one isomer is biologically active - Functional groups are the components of organic molecules that are most commonly involved in chemical reactions
■ Number and arrangement of functional groups give each molecule its unique properties
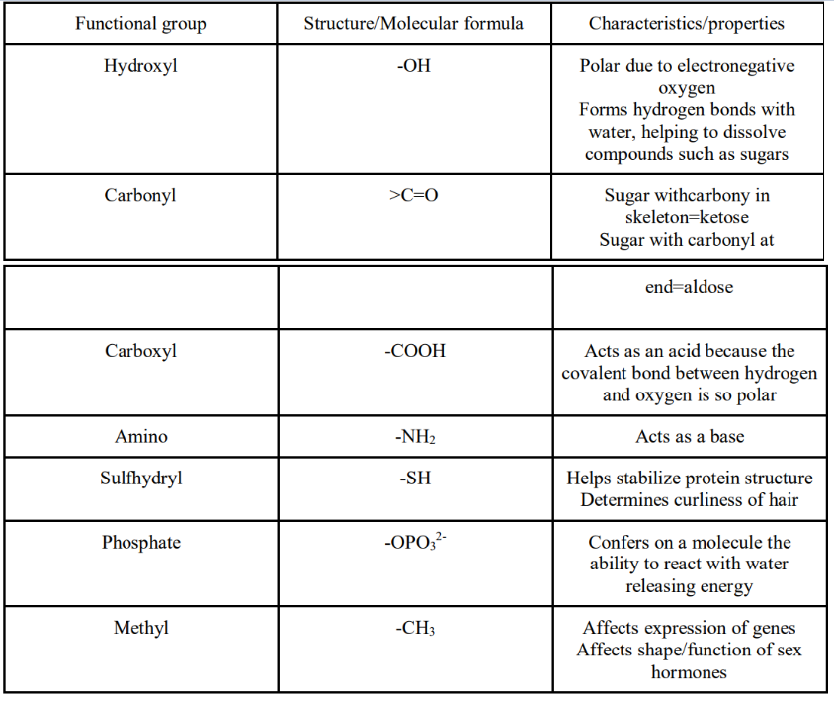
➢ Most macromolecules are chains of building blocks called polymers. The individual building blocks of a polymer are called monomers
➢ Carbohydrates
- Contain carbon, hydrogen, and oxygen in a 1:2:1 ratio
- Monosaccharides
■ Most common are glucose and fructose
● Glucose-
-
- Most abundant
- Part of food humans eat
- Made by plants during photosynthesis
■ Broken down to release energy
● Fructose-
-
- Common sugar in fruits
-
-
-
-
● Can be depicted as either straight or rings

■ 6 carbon-sugars
● Formula: $\mathrm{C}_6\mathrm{H}_{12}\mathrm{O}_6$
- Disaccharides
■ 1 monosaccharide+1 monosaccharide=1 Disaccharide
■ Formed by dehydration synthesis
● Aka condensation
● Hydrogen (-H) from one sugar combines with hydroxyl group (-OH) of another sugar molecule to create water as byproduct

● Bond is called glycosidic linkage
■ Broken apart by hydrolysis
● Reverse of dehydration
● Water is used to break apart glycosidic linkage
○ Polysaccharides
■ Repeated units of monosaccharides
■ Most common
● Starch
- Stores sugar in plants
- Made up of alpha-glucose molecules
● Cellulose - Made up of $\beta$-glucose molecules
- Chitin
■ Structural molecule in walls of fungi/arthropod exoskeletons
■ Used as surgical thread since it breaks down in body
● Glycogen
- Stores sugar in animals
➢ Proteins
- Amino acids=monomer of proteins
■ 20 kinds of naturally occurring amino acids - Contain:
■ Carbon
■ Hydrogen
■ Oxygen
■ Nitrogen - 4 parts of an amino acid centered around a central carbon
■ Amino group $(-NH_2)$
■ Carboxyl group (-COOH)
■ Hydrogen
■ R group
● Aka side chain
● Interchangeable
● Vary in composition, polarity, charge, shape depending on specific side chain
● Polar R groups point outward, hydrophobic R groups point inward - Polypeptides
■ Amino acid + amino acid= dipeptide
● Formed by dehydration synthesis
● Bond is called a peptide bond
● Multiple amino acids= polypeptide - Once a polypeptide chain twists and folds on itself, it forms a $\text3D$ structure called a protein

- Higher protein structure (4 levels total)
■ Primary structure
● Linear sequence of amino acids
● Covalent (peptide) bonds
■ Secondary structure
● Protein beings to twist–2 options
- Forms a coil (alpha-helix)
- Zigzagging pattern (known as beta-pleated sheets)
● Shape depends on R-group
● Formed by amino acids that interact with other amino acids closeby in the primary structure
● Hydrogen bonds between carbonyl and amino group
● Interactions between amino and carboxyl groups of protein backbone
● After secondary structure forms, formerly distant amino acids are now closeby–tertiary structure can form
■ Tertiary structure
● Can be both alpha and beta helix/sheets within structure
● Covalent disulfide bridge often stabilizes structure
● Bonds between R groups - Hydrogen bonds
- Ionic bonds
- Disulfide bridges
- Hydrophobic interactions
■ Quaternary structure
● Several different polypeptide chains sometimes interact with each other
● Same bonds as above, but between peptide chains rather than between R
groups
■ Mistakes in structure can denature a protein
● Change of shape=change of function - Ex. pH or heat can denature protein
■ Protein folding can involve chaperone proteins (chaperonins)
● Help protein fold properly
● Make process more efficient

➢ Lipids
- Like carbs, consist of carbon, hydrogen and oxygen, but not in a fixed ratio
- Do not form polymers
- Little-no affinity for water
■ Hydrophobic due to nonpolar covalent bonds of hydrocarbon - Common examples:
■ Triglycerides
● Glycerol molecule+3 fatty acid chains attached - Fatty acid chain is mostly a long chain of carbons where each carbon is covered in hydrogen; One end of the chain has a carboxyl group $(-COOH)$
■ Vary in length and /location(s) of double bonds - Glycerol is a 3-carbon alcohol with a hydroxyl group attached to each carbon
● Fats separate from water because water forms hydrogen bonds with itself while excluding the fats
● In order to be made, each of the carboxyl groups of the 3 fatty acids must react with one of the 3 hydroxyl groups of the glycerol molecule via dehydration synthesis - bond=ester linkage
● Saturated fatty acid - No double bond
- Carbon chain completely filled (“saturated”) with hydrogen
- Usually solid at room temp.
● Unsaturated fatty acid - Double bond along carbon chain, causing a bend
■ Bend allows triglyceride to become LESS dense, making it liquid at room temperature - Polyunsaturated fatty acid has multiple double bonds within the fatty acid, causing many bends

■ Phospholipids
